2022年1月の記事一覧
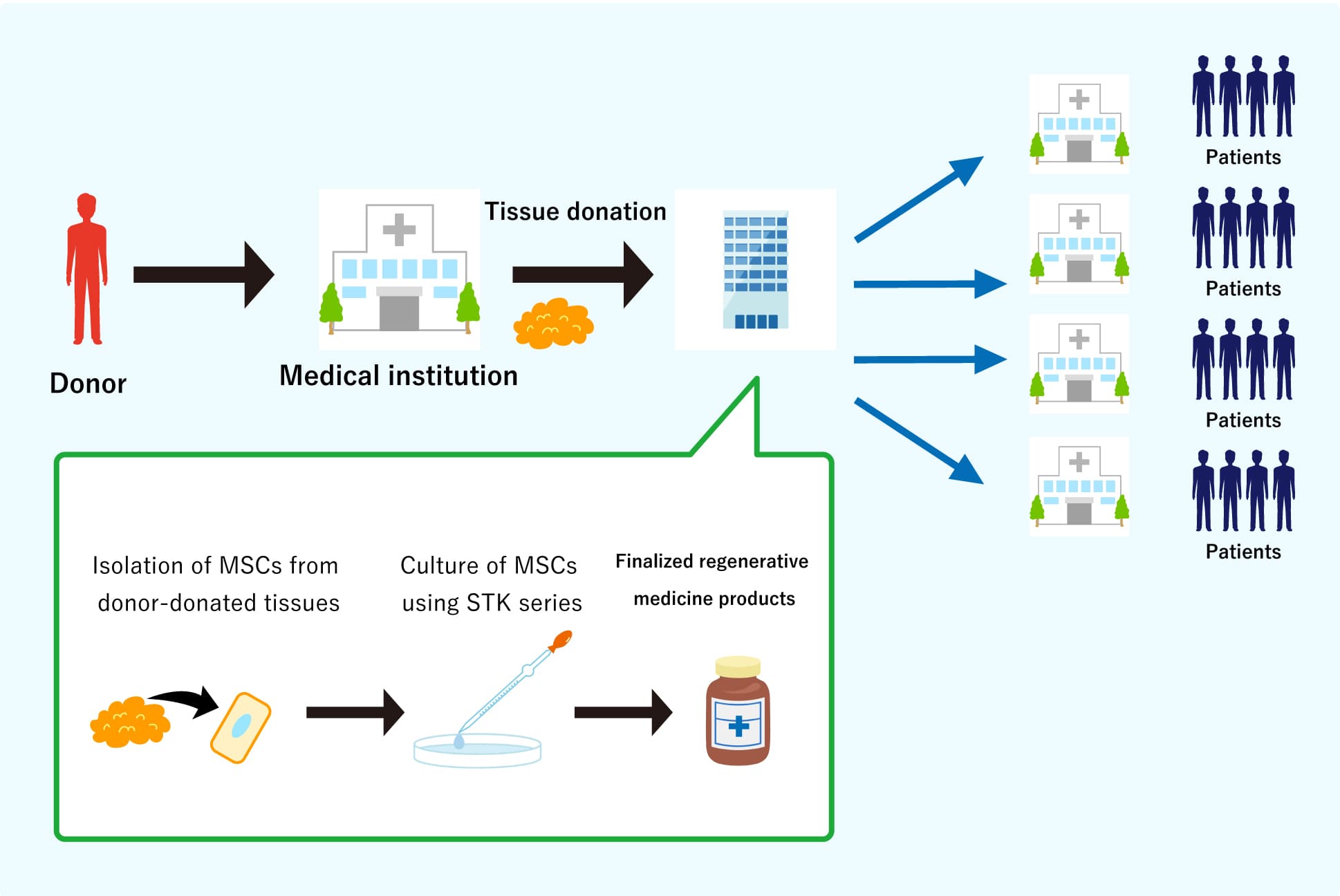
Cells used in regenerative medicine are traditionally cultured with autologous human serum (the patient's own serum) or fetal bovine serum (FBS). However, serum components vary widely from one to another, and they significantly affect the quality of the cultured cells, such as proliferation capability. In addition to their source variety, in the case of FBS, other risks of pathogen contamination, such as animal-derived allergic protein, or certain viruses which infect humans, are also there. We think it would be ideal for conducting cell culture in serum-free conditions to eliminate these risk factors, then we have developed the serum-free media STK® series for MSCs.
The chemically defined cell culture media of the STK® series enable us to cultivate MSCs without using any serum and produce high-quality MSCs more stably.
We have carefully selected ingredients to decide the compositions of STK® series, instead of using any serum.
- Serum-free medium for
primary mesenchymal stem cells - Serum-free medium specialized for primary culture of mesenchymal stem cells from bone marrow, adipose (fat), synovial membrane, and other tissues.
This unique medium helps MSCs proliferate selectively. - Serum-free medium for mass expantion of mesenchymal stem cells
- Serum-free medium for proliferation and culture of mesenchymal stem cell expansion.
In combination with STK®1, STK®2 enhance the MSCs’ potentials (e.g. growth ratio, differentiation efficacies, and other functional properties). - Serum-free medium for osteogenic
differentiation of mesenchymal stem cells - Highly efficient in inducing osteogenic differentiation within only one week.
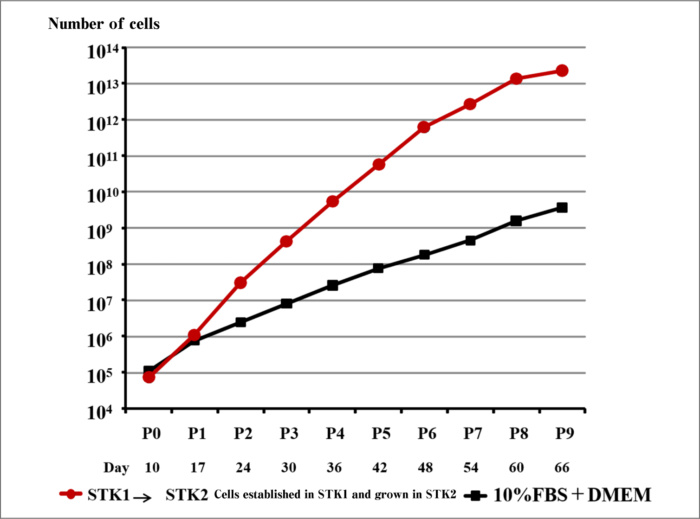
More than 1000 times the growth rate can be accomplished compared to conventional serum-supplemented media.
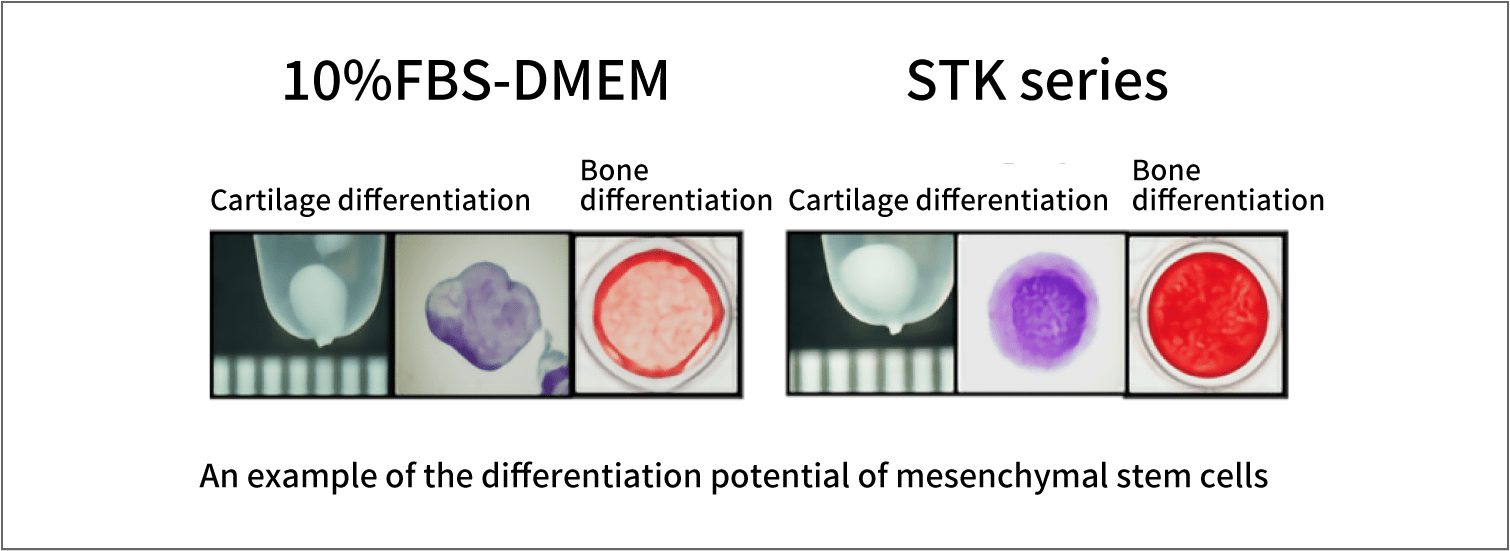
STK series also enables MSCs to maintain their differentiation potentials, as well as their growth performance.
We will supply regenerative medical products with stable quality, and patients will be able to access high quality and continuous treatment at wide variety of medical institutions.
We keep on engaging in the business development to provide regenerative medicine to any people suffering from disease without complete cure. We call our MSCs as "gMSC®," represents “guaranteed” MSC, which is produced from allogeneic tissue using TWOCELLS’ originally developed serum-free media (STK® series) and culture technologies.

In comparison with the transplantation of autologous and allogeneic products in regenerative medicine, allogeneic transplantation is considered to be more suitable.
Our currently developing pipelines for allogeneic regenerative medical products are described here.
We act with pride as members of TWOCELLS.
1.Management Philosophy
We aim to become "a global company of the 21st century that contributes to world’s healthcare and people's health through regenerative medicine."
In other words, there are four pursuits.
- (1) Pursue humanity (Fighting against diseases)
- (2) Pursue sociality (Prevail in the society)
- (3) Pursue economic efficiency (Contribute to the healthcare economy)
- (4) Pursue internationality (Act globally)
2. Message
We are a venture business company dedicated to regenerative medicine technologies to utilizing Mesenchymal Stem Cells (MSCs), which are taken from an adult body.
Since we have started our business, when regenerative medicine is not well-known, we have been engaging in developments of regenerative medicine products with MSCs for more than 20 years.
Our team have common passion (“Kokorozashi” in Japanese), which lies in “provide regenerative medicine as novel treatment options for patients with unmet medical needs.
Our Phase 3 clinical trials of gMSC1 ended in suggesting efficacy by structural evaluations with MRI and arthroscopy, although its primary endpoint failed in significance.
We of course assume that it not so easy to get an approval, for the possibility to be needed to obtain additional clinical data to discuss with regulatory authorities.
Even though, we keep on trying development of gMSC®1 to fulfill the unmet medical needs (UMNs) with our “Kokorozashi,” aiming to realize our management philosophy to become "a global company of the 21st century that contributes to world’s healthcare and people's health through regenerative medicine."
To realize our “Kokorozashi” with business growth and corporate independence, we tackle our missions.
3. Business Policy
TWOCELLS aims to be a value-creation company with "Integrated R・D・M Systems" that unitarily include Research, Development, and Marketing of MSCs-derived regenerative medicine products.
4. Meanings of the Corporate Logo

The TWOCELLS’ logo symbolizing “two cells” and “infinity” represents two founders who established TWOCELLS, and the limitless possibilities of medical technologies and the great hope that they bring.
Company Profile
| Company Name | TWOCELLS COMPANY, LIMITED |
|---|---|
| Date of Establishment | April 23, 2003 |
| Office/Laboratory | 2nd Floor, Otani Building, 1-6-10 Deshio, Minami-ku, Hiroshima 734-0001, Japan |
| Paid-in Capital | \ 100 million (as of March 31, 2025) |
| Directors and Corporate Auditors | Masaya Matsumoto, Representative Director Shinichi Hasegawa, Director Minoru Tsukamoto, Director Yosuke Yamane, Accounting Advisor |
| Business activities | R&D, manufacturing, and marketing(sales) of regenerative medical products and other related products (e.g. diagnostics, reagents, medical devices, etc.) |
History
-
April 2003
Established TWOCELLS COMPANY, LIMITED in Minami-ku, Hiroshima, as a startup company from Hiroshima University -
August 2006
Registered trademark of "gMSC®" (registration number 4987485) -
May 2008
Started sales of serum-free medium STK®2 -
October 2009
Patent granted for serum-free medium for research (Patent No. 4385076),
supported by grants from JST (Japan Science and Technology agency) -
February 2010
Started sales of serum-free medium STK®1 -
September 2010
Started sales of serum-free medium STK®3 -
January 2014
Established gMSC Center, a facility for developing formulation technology, supported by grants from NEDO (New Energy and industrial technology Development Organization) -
March 2015
Developed clinical-grade serum-free medium STK®1 and STK®2 -
August 2015
Osaka Univ. opened the MSCs Cell Bank as an achievement of Joint research with TWOCELLS -
April 2016
License agreement signed with Chugai Pharmaceutical for the knee cartilage regenerative cell product “gMSC®1” -
May 2017
Submitted phase III clinical trial of knee cartilage regenerative medicical product gMSC®1 (IND) -
October 2021
Completed case registration of phase III clinical trial of gMSC®1 -
April 2023
Announced the termination of license agreements about gMSC®1 with Chugai Pharmaceuticals -
November 2023
Relocation of the head office -
June 2025
Annouced a License Agreement with Kaken Pharmaceutical Co., Ltd. for “gMSC®1”, the Allogenic Synovial Mesenchymal Stem Cell-Derived Three-Dimensional Artificial Tissue
Access
Office/Laboratory
2nd Floor, Otani Building, 1-6-10 Deshio,
Minami-ku, Hiroshima 734-0001, Japan
TEL: +81-82-256-2451
FAX: +81-82-258-1012
Businesses
To realize our management philosophy, we engage in the R&Ds, manufacturing and marketing of regenerative medicine and its related products.
R&Ds
TWOCELLS is conducting research and development to provide high-quality MSC products by applying originally developed cell culture technology using serum-free media.
As a typical example, gMSC®1 and other pipelines, are introduced on the following pages.
"gMSC®" represents “guaranteed” MSC, which is produced from allogeneic tissue using TWOCELLS’ originally developed serum-free media and culture technologies.
Regenerative Medicine
“Regenerative medicine” is a medical treatment that aims to recover the functions of living tissues or organs with dysfunctions, by utilizing cells and artificial materials actively.
Currently, regenerative medicine with various cell types is being studied. TWOCELLS is focusing on using mesenchymal stem cells (hereinafter referred to as “MSCs.”) In this case, human MSCs are expanded in vitro (outside the body) and then transplanted into the patient's body.

MSCs
MSCs stand for mesenchymal stem cells. MSCs are one of the adult stem cells found in human bone marrow, adipose tissue (fat), the umbilical cord, and the synovium (tissue around joints).
It can be differentiated into bone, cartilage, tendon, adipocytes, and nerve cells.
Because of its differentiation capacity, the number of patients with diversified disease profiles treated by MSCs transplantation is expected to be very large.
In addition to their differentiation capacities, MSCs have immunomodulative activities like secretion of anti-inflammatory cytokines.
Because MSCs are the stem cells found in adults, they have the advantage of lower bioethical hurdles than embryonic stem cells (ES cells), which use embryos. It also has the advantage of being safer than the ES cells or iPS cells because cancer risk with MSCs is lower than with other cell types.
However, the abundance of MSCs is only 1 in 10000 ~ 100000 bone marrow cells, and it further decreases with age.
Therefore, obtaining a large amount of bone marrow fluid is necessary to get enough MSCs for transplantation.
In addition, during conventional in vitro culture of MSCs using the human and bovine serum, the proliferation capacity and pluripotency of MSCs are likely to be decreased, making it difficult to secure sufficient the number of cells required for treatment.
Therefore, TWOCELLS has developed a unique culture method (using the STK series serum-free media for MSCs) that dramatically increases the proliferation of MSCs while maintaining their differentiation potentials.
In this way, TWOCELLS continues various research and development projects to develop and disseminate “regenerative medicine using MSCs.”