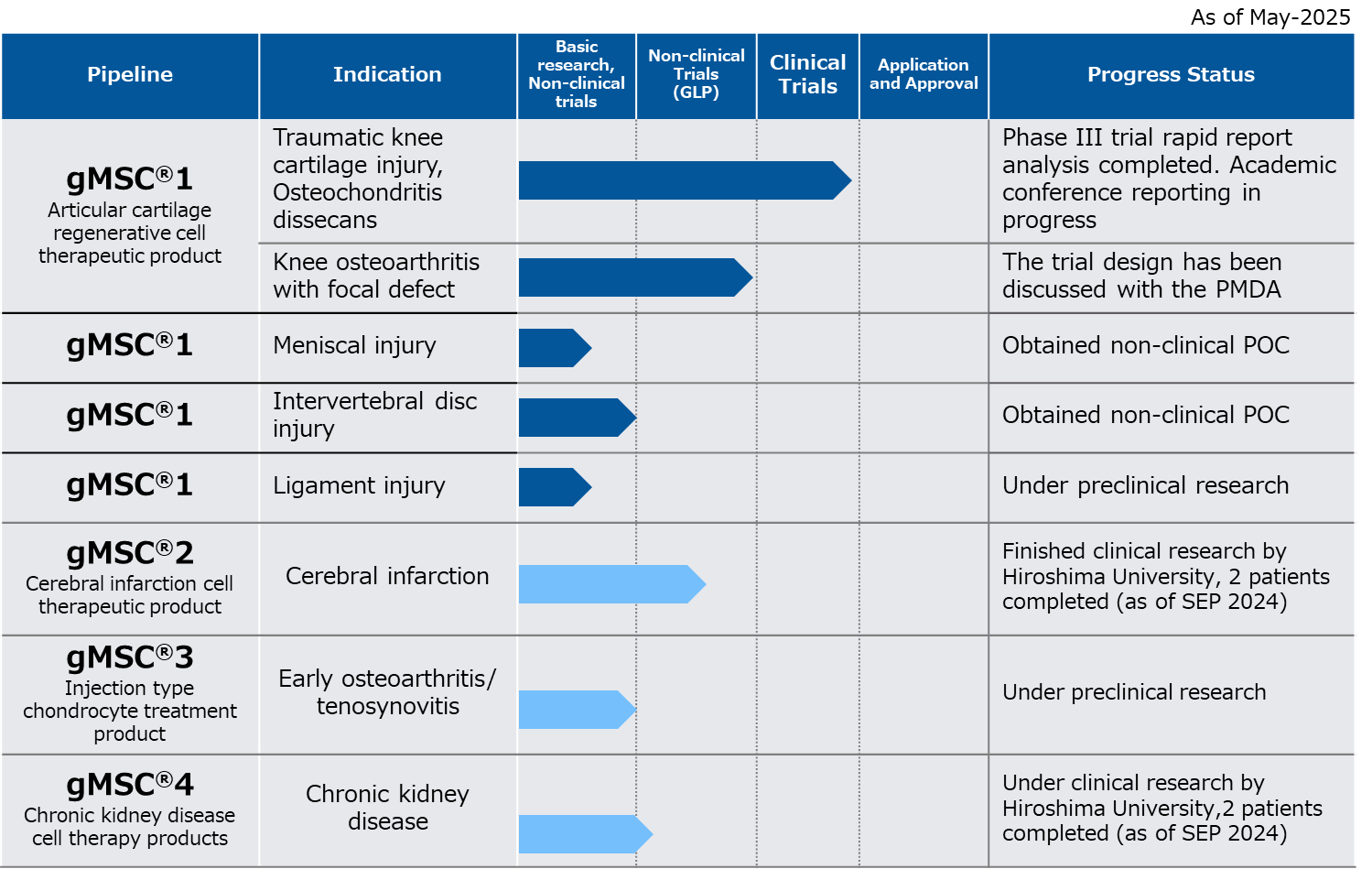
Pipelines
Based on two development concepts, TWOCELLS is working on developing regenerative medical products.
Use of Allogeneic Mesenchymal Stem Cells
By processing and transplanting allogenic cells,
- Products can be Stocked to allow people to use the product immediately when treatment is needed. In addition, there is no need for "surgery to collect tissue from the patient's own body," which is necessary for autologous transplantation (treatment using the patient's own cells.)
- Since the quality can be checked beforehand, there is no "product quality defect due to donor/source variability," which often happens in autologous transplantation.
- It is easy to reduce product costs through mass production and supply because it can be manufactured for multiple people at once.
Culture Process Using Serum-Free Media, STK® Series
By culturing with TWOCELLS’ serum-free media, we maximize MSCs’ capacity, thereby increasing production capacity for mass production, leading to cost reductions.
In addition, according to our authorized quality standards, high-quality MSCs can be stably produced.
The pipelines currently under development are as follows.