gMSC®1
gMSC®1 is a regenerative medical product for damaged knee cartilage using allogeneic MSCs, currently under development.
In addition, once a joint cartilage is damaged and if the damage exceeds, it may cause osteoarthritis because it can not be healed spontaneously.
TWOCELLS is first focusing on developing products for knee damaged cartilages.
An image of gMSC®1
"TEC" stands for "Tissue-Engineered Construct," and was first developed by Osaka University.
It can create a three-dimensional, flexible shape by itself, without requiring any artificial scaffolding materials (scaffolds) for transplanted cells, and is devoid of safety concerns.
The significant technological advancement of gMSC®1 includes the use of TEC production technology to produce transplantation constructs by serum-free culturing of MSCs derived from allogeneic synovium.
1. Technologies for serum-free culture of MSCs, dedicated serum-free media (STK®1, STK®2) and techniques using these
2. Formulation technologies of “TEC”
3. High quality cryopreservation technology for “TEC” (three-dimensional cellular construct)
4. Knowledge for GCTP-grade process development and manufacturing, including QC package
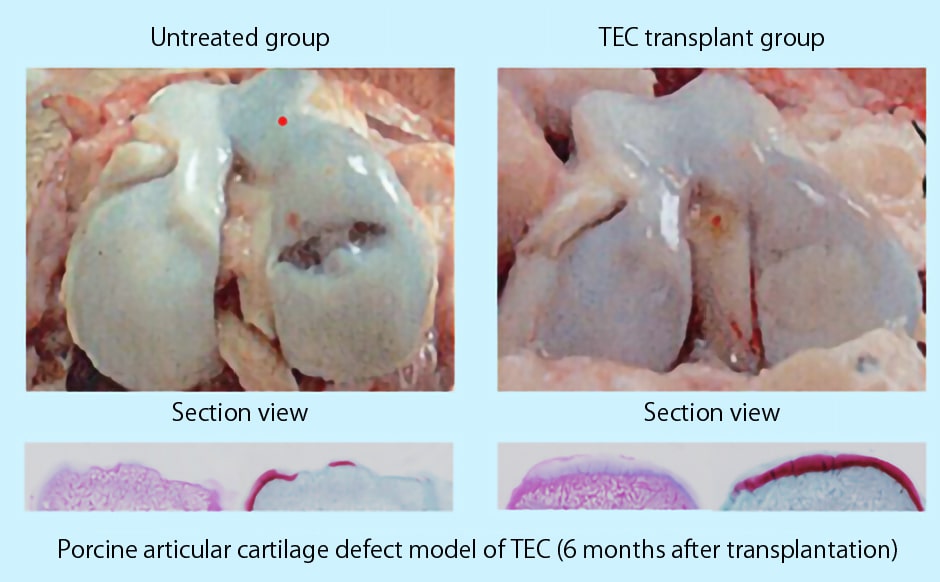
When TEC was transplanted into a porcine articular cartilage defect model, as shown in Figure1, TEC was fixed to the defect site.
Significant cartilage regeneration was observed without any prominent adverse effects.
Clinical studies conducted at Osaka University have confirmed the safety and efficacy of TEC in human(1. (When TEC was transplanted to subjects with knee cartilage damage, it was observed that the damaged knee cartilage was covered with cartilage-like tissue, 48 weeks after transplantation, as shown in Figure2.)
 Picture 1
Picture 1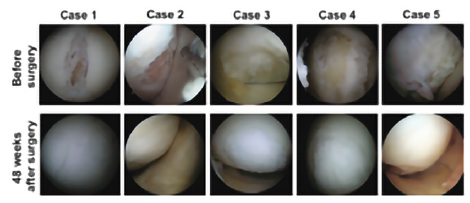 Picture 2
Picture 2
Data provision:
Prof. Dr. Norimasa Nakamura,
Osaka Health Science University, institute for Medical Science in Sports
Osaka University global center for the medical engineering and informatics
(1: Kazunori S., Norimasa N. et al., The American Journal of Sports Medicine, 2018 Aug; 46 (10): 2384 -2393.)
TWOCELLS' original serum-free culture technology enables the mass production of high-quality products by culturing allogeneic MSCs. If it is proven to be effective through clinical developments, many people can be treated in the future.
In addition, we have developed a fundamental cryopreservation technology of gMSC®1 to improve the ease of handling in manufacturing, distribution, and medical settings.

