2022年1月の記事一覧
Based on two development concepts, TWOCELLS is working on developing regenerative medical products.
Use of Allogeneic Mesenchymal Stem Cells
By processing and transplanting allogenic cells,
- Products can be Stocked to allow people to use the product immediately when treatment is needed. In addition, there is no need for "surgery to collect tissue from the patient's own body," which is necessary for autologous transplantation (treatment using the patient's own cells.)
- Since the quality can be checked beforehand, there is no "product quality defect due to donor/source variability," which often happens in autologous transplantation.
- It is easy to reduce product costs through mass production and supply because it can be manufactured for multiple people at once.
Culture Process Using Serum-Free Media, STK® Series
By culturing with TWOCELLS’ serum-free media, we maximize MSCs’ capacity, thereby increasing production capacity for mass production, leading to cost reductions.
In addition, according to our authorized quality standards, high-quality MSCs can be stably produced.
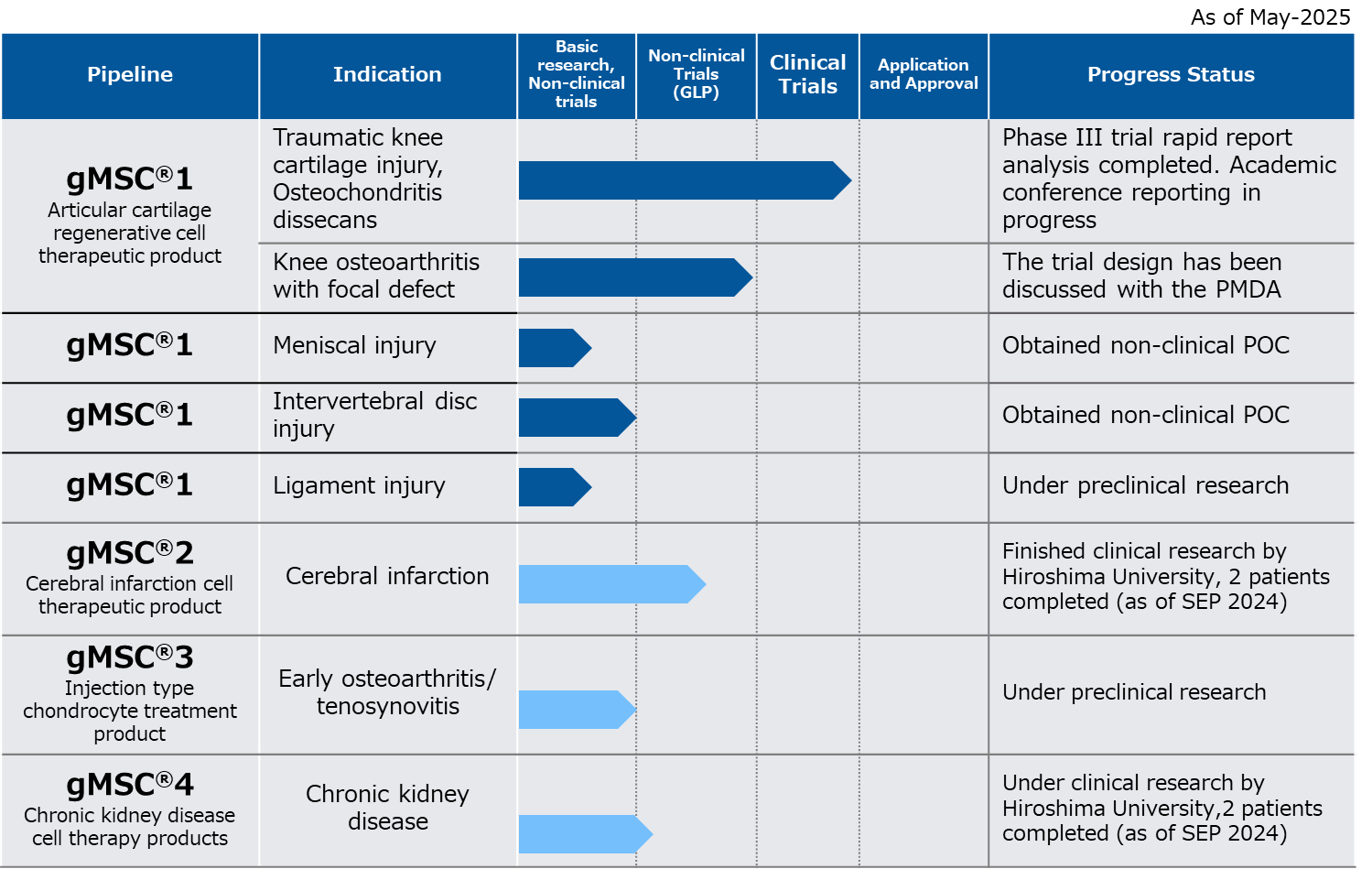
The pipelines currently under development are as follows.
gMSC®1 is a regenerative medical product for damaged knee cartilage using allogeneic MSCs, currently under development.
In addition, once a joint cartilage is damaged and if the damage exceeds, it may cause osteoarthritis because it can not be healed spontaneously.
TWOCELLS is first focusing on developing products for knee damaged cartilages.
An image of gMSC®1
"TEC" stands for "Tissue-Engineered Construct," and was first developed by Osaka University.
It can create a three-dimensional, flexible shape by itself, without requiring any artificial scaffolding materials (scaffolds) for transplanted cells, and is devoid of safety concerns.
The significant technological advancement of gMSC®1 includes the use of TEC production technology to produce transplantation constructs by serum-free culturing of MSCs derived from allogeneic synovium.
1. Technologies for serum-free culture of MSCs, dedicated serum-free media (STK®1, STK®2) and techniques using these
2. Formulation technologies of “TEC”
3. High quality cryopreservation technology for “TEC” (three-dimensional cellular construct)
4. Knowledge for GCTP-grade process development and manufacturing, including QC package
When TEC was transplanted into a porcine articular cartilage defect model, as shown in Figure1, TEC was fixed to the defect site.
Significant cartilage regeneration was observed without any prominent adverse effects.
Clinical studies conducted at Osaka University have confirmed the safety and efficacy of TEC in human(1. (When TEC was transplanted to subjects with knee cartilage damage, it was observed that the damaged knee cartilage was covered with cartilage-like tissue, 48 weeks after transplantation, as shown in Figure2.)
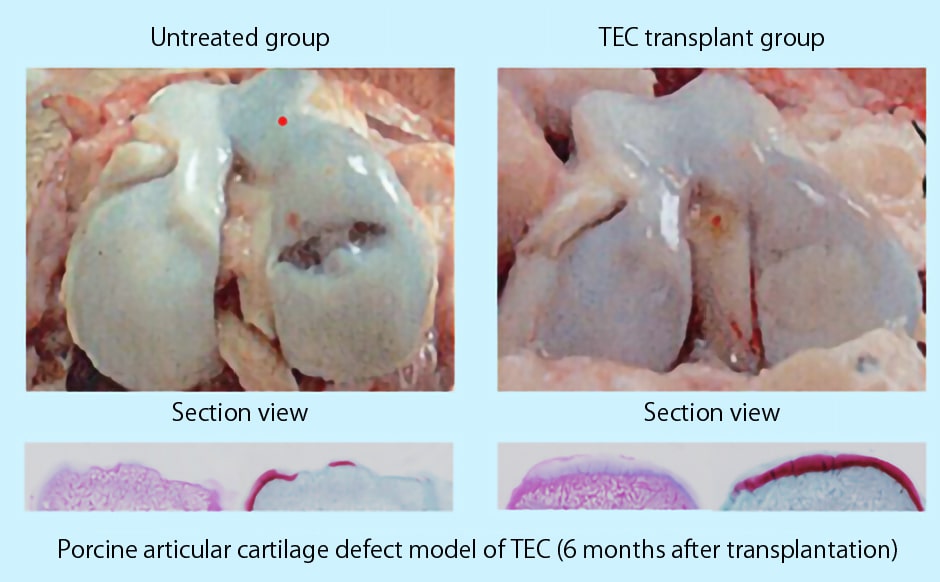 Picture 1
Picture 1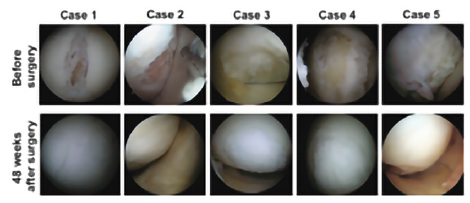 Picture 2
Picture 2
Data provision:
Prof. Dr. Norimasa Nakamura,
Osaka Health Science University, institute for Medical Science in Sports
Osaka University global center for the medical engineering and informatics
(1: Kazunori S., Norimasa N. et al., The American Journal of Sports Medicine, 2018 Aug; 46 (10): 2384 -2393.)
TWOCELLS' original serum-free culture technology enables the mass production of high-quality products by culturing allogeneic MSCs. If it is proven to be effective through clinical developments, many people can be treated in the future.
In addition, we have developed a fundamental cryopreservation technology of gMSC®1 to improve the ease of handling in manufacturing, distribution, and medical settings.
新年のご挨拶
新年、明けましておめでとうございます。
関係各位の皆様には昨年も大変お世話になりました。
皆様の長年のご支援により第III相比較臨床試験は、2017年11月最初の患者さんから4年の歳月を経て目標の症例登録完了することができました。
社員一同、心より御礼申し上げます。
本年も再生医療を通じて世界の医療や人々の健康に貢献するという理念を実現するため全社一丸となって邁進してまいりますので、より一層のご支援、お引立てを賜りますようお願い申し上げます。
皆様のご健康とご多幸をお祈りし、新年のご挨拶とさせていただきます。
本年も宜しくお願い申し上げます。
株式会社ツーセル
代表取締役社長
日浦 敏樹